Tracking nitrate and ammonium in the environment
Newly developed methodologies are improving our understanding of nitrogen cycling.
20/10/2022
At BGS’s Stable Isotope Facility, we are developing a suite of laboratory methodologies to analyse the key nitrogen-bearing species nitrate and ammonium. These methods will allow much lower concentration samples to analysed, helping improve our understanding of nitrogen cycling within the environment.
The nitrogen cycle
Alongside carbon and phosphorus, nitrogen is one of the key micronutrients critical to all life on Earth. It is a core component in amino acids, which, in turn, form the building blocks of all genetic material: DNA and RNA.
Nitrogen is the most abundant gas on Earth, making up 78 per cent of the air we breathe. Atmospheric nitrogen is converted by bacteria in the soil to ammonium (NH4+), in a process called nitrogen fixation. From here it is converted to nitrite (NO2–) and nitrate (NO3–), which can be taken up by plants, thus entering the food chain.
In addition to natural nitrogen fixation, humans apply nitrogen-rich fertilisers to soils to reduce natural nitrogen limitation and promote crop productivity.
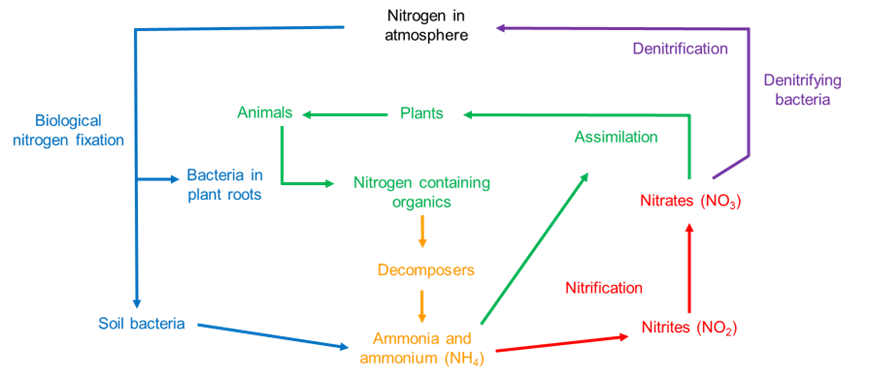
The nitrogen cycle, showing nitrogen fixation from nitrogen in the atmosphere to ammonium and the conversion of ammonium to nitrate before it is taken up into plants. Denitrification is the main process by which nitrogen is then lost back into the atmosphere. BGS © UKRI.
This application of fertilisers can have negative consequences. When nutrient-rich water runs off arable land and enters surface waters, for example lakes or rivers, it can promote uncontrollable algal growth. This can drastically decrease water quality, reducing the water’s oxygen content and leading to the death of aquatic species. Additionally, high concentrations of nitrogen can enter groundwater, from which it is almost impossible to remove, polluting aquifers that are traditionally used for water extraction and human consumption.
It is therefore critical that we understand how nitrogen enters our most precious environments, how it is cycled, transformed between different nitrogen species and released back into the atmosphere. To understand these processes, we need effective ‘tracers’. One of these is the stable isotope composition of nitrogen within different key molecules, including nitrate and ammonium.
New methods
The Stable Isotope Facility has carried out stable isotope analysis of nitrate and ammonium for many years. Traditional methods convert dissolved nitrate or ammonium to a solid, for example silver nitrate, allowing for the combustion of this solid within an elemental analyser coupled to a mass spectrometer. However, these methods required large volumes of sample water (more than 1 l) and high concentrations of either nitrate or ammonium. This used to limit the types and numbers of samples we could realistically analyse.
Recently, we have been working to improve in line with recent published methods. These new methods are up to a thousand times more sensitive than our old procedures, meaning we can analyse much smaller volumes of samples (1 to 4 ml) and at much lower concentrations (less than 1 mg-l). Unlike our old methods, these new techniques convert the dissolved nitrate or ammonium to nitrous oxide gas, which is then analysed by our trace gas and mass spectrometer system (Sercon Cryogas HS2022). This new mode of analysis has needed a lot of setting up and testing over the past year, but we are now getting great data for low concentration nitrogen species.
The Sercon and BGS team, who developed the Cryogas system currently used for N2O and other trace gas stable isotope analysis at BGS. BGS © UKRI.
The hope is that this faster, cheaper and more sensitive analysis will promote more wide-ranging nitrogen cycling studies using stable isotopes, helping to answer some of the key questions surrounding nitrogen pollution and sustainable fertilisation.
Acknowledgment
Thanks goes to the BGS Innovation Fund and Sercon for supporting this work.

Dr Andrew Smith
Isotope geochemist
Relative topics
Latest blogs

AI and Earth observation: BGS visits the European Space Agency
02/07/2025
The newest artificial intelligence for earth science: how ESA and NASA are using AI to understand our planet.

Geology sans frontières
24/04/2025
Geology doesn’t stop at international borders, so BGS is working with neighbouring geological surveys and research institutes to solve common problems with the geology they share.

Celebrating 20 years of virtual reality innovation at BGS
08/04/2025
Twenty years after its installation, BGS Visualisation Systems lead Bruce Napier reflects on our cutting-edge virtual reality suite and looks forward to new possibilities.

Exploring Scotland’s hidden energy potential with geology and geophysics: fieldwork in the Cairngorms
31/03/2025
BUFI student Innes Campbell discusses his research on Scotland’s radiothermal granites and how a fieldtrip with BGS helped further explore the subject.

Could underground disposal of carbon dioxide help to reduce India’s emissions?
28/01/2025
BGS geologists have partnered with research institutes in India to explore the potential for carbon capture and storage, with an emphasis on storage.

Carbon and oxygen isotope analysis of carbonates and the development of new reference materials
18/12/2024
Dr Charlotte Hipkiss and Kotryna Savickaite explore the importance of standard analysis when testing carbon and oxygen samples.

Studying oxygen isotopes in sediments from Rutland Water Nature Reserve
20/11/2024
Chris Bengt visited Rutland Water as part of a project to determine human impact and environmental change in lake sediments.

Celebrating 25 years of technical excellence at the BGS Inorganic Geochemistry Facility
08/11/2024
The ISO/IEC 17025 accreditation is evidence of technical excellence and reliability, and a mark of quality assurance.

Electromagnetic geophysics in Japan: a conference experience
23/10/2024
Juliane Huebert took in the fascinating sights of Beppu, Japan, while at a geophysics conference that uses electromagnetic fields to look deep into the Earth and beyond.

Exploring the role of stable isotope geochemistry in nuclear forensics
09/10/2024
Paulina Baranowska introduces her PhD research investigating the use of oxygen isotopes as a nuclear forensic signature.

BGS collaborates with Icelandic colleagues to assess windfarm suitability
03/10/2024
Iceland’s offshore geology, geomorphology and climate present all the elements required for renewable energy resources.

Mining sand sustainably in The Gambia
17/09/2024
BGS geologists Tom Bide and Clive Mitchell travelled to The Gambia as part of our ongoing work aiming to reduce the impact of sand mining.





