BGS laboratory spotlight: isotopes as recorders of climate and environmental change
How measuring oxygen and carbon isotopes in tiny fossils improves our understanding of past climate.
06/09/2023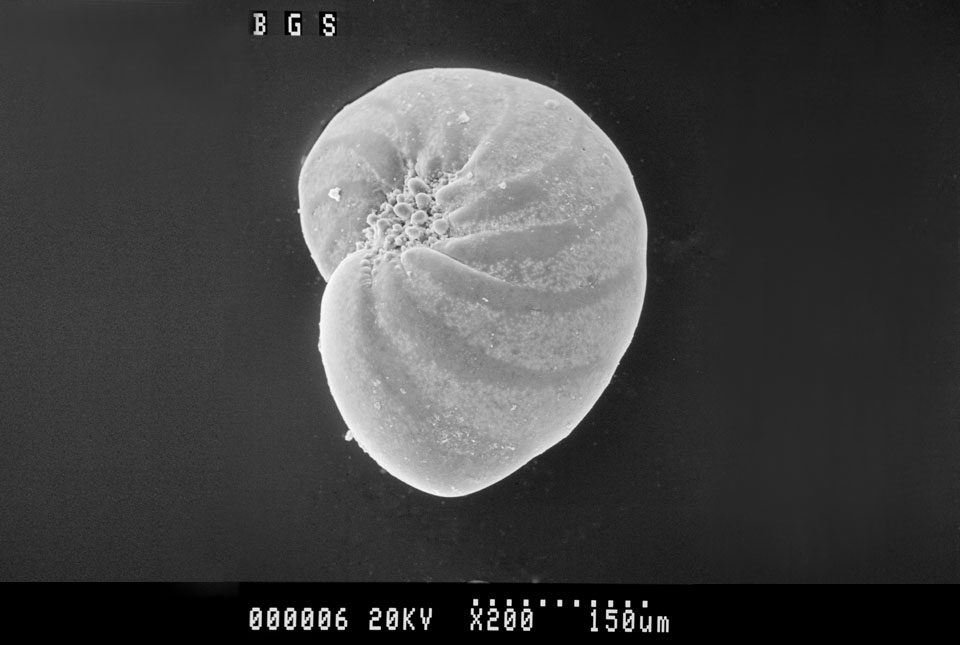
The National Environmental Isotope Facility (NEIF), a UKRI/NERC facility hosted at BGS Keyworth, recently took delivery of a new isotope-ratio mass spectrometer for measuring oxygen and carbon isotopes in very small (microgram-scale) samples of carbonate from fossil material. This instrument is helping scientists answer fundamental questions about climate and environmental change.
Evidence published in the latest assessment report from the Intergovernmental Panel on Climate Change confirms that human activity is warming our planet. The rate and scale of environmental change in response to warming is rapid, widespread and unprecedented over past centuries. Gaining a longer-term perspective on the types, rates and causes of previous climactic and environmental change, beyond the instrumental record, is crucial to enable us to make accurate predictions about the effects on our climate in the future.
A vital component of understanding past climates is the generation of robust data, which enables scientists to investigate the response of environments to climate change across thousands to millions of years of Earth’s history. Palaeoclimate archives — for example, ocean and lake sediments, cave deposits and corals — can be used to reconstruct how environments around the world have been shaped by climate change in the geological past. This provides essential information to better project future climate pathways.

Elementar IsoPrime100 precision dual inlet stable isotope-ratio mass spectrometer used for analysis of carbon and oxygen isotopes in calcium carbonate. BGS © UKRI
How can isotopes help to unravel the history of past environmental and climate change?
Let’s take oxygen (O) as an example. Over 99.7 per cent of oxygen atoms contain eight protons and eight neutrons, giving oxygen a total atomic mass of 16. However, a very small amount (around 0.2 per cent) of oxygen atoms have two additional neutrons, resulting in a heavier mass of 18. These are the two main stable isotopes of oxygen, known as 16O and 18O.
Water molecules contain one oxygen and two hydrogen (H) atoms: H2O. Through natural processes, for example when water evaporates from the ocean as part of the global water cycle, the difference in atomic mass between 16O and 18O causes a measurable change in the proportion of 18O compared to 16O. Water molecules that contain H218O are heavier and more difficult to evaporate. A preferential transfer of H216O from ocean to atmosphere occurs, leaving the ocean with more H218O.
When the evaporated water is transported to polar regions and falls as rain or snow, it becomes trapped in ice; the 16O is locked away and does not return to the ocean. Over geological time, the build-up of large ice sheets captures ever-increasing amounts of 16O, resulting in the global ocean having relatively more 18O. Whilst we can’t measure the isotope changes that take place over thousands to millions of years directly, we can use natural materials that contain ‘snapshots’ of the oxygen isotope ratio of the ocean from different points in geological time.
Some marine organisms grow shells while they are alive. When an individual dies, its shell can be preserved in sediments on the ocean floor, unlike the soft parts of its body that decay following death. The shell is made from calcium carbonate (CaCO3) and the amount of 18O and 16O incorporated into the shell is linked directly to the balance of oxygen isotopes present in the ocean water during the organism’s lifetime. Over time, many of these shells accumulate on the sea floor, layer upon layer, capturing the relative proportion of 18O compared to 16O in the ocean. If the oxygen isotope composition of the ocean water changes (for example, due to a different global climate state), the shift is captured in the shells and reflected in the sediments.
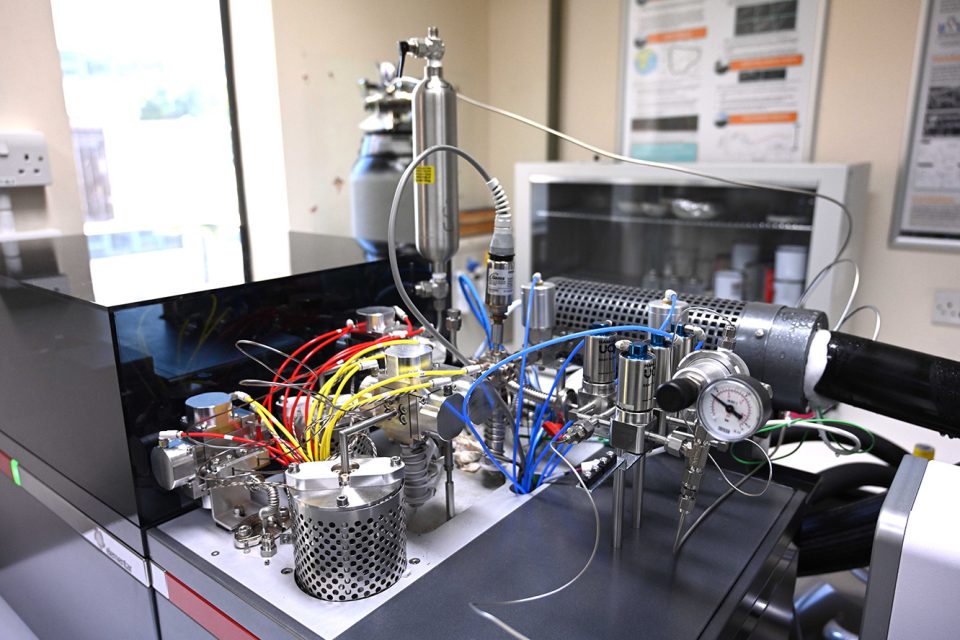
A close-up of the inlet on mass spectrometer. Frost is forming around the internal ‘cold finger’, which is in the process of freezing a sample of gas to remove impurities. BGS © UKRI
We can extract, clean and measure the oxygen isotopes in fossilised shells found in cores of time-ordered sequences of marine sediment. These measurements help us reconstruct how the oxygen isotopes and, by extension, climate has evolved over time. This is known as a ‘proxy’ record, because the oxygen isotope composition of marine shells is used as a proxy for changes in global climate conditions.
Measuring materials at the micro-scale for oxygen isotopes
Isotope-ratio mass spectrometers allow us to measure very precisely the relative proportion of different isotopes of a particular element in a wide range of materials. In natural archives, this can be for the purpose of interpreting past climate and environmental change.

Kotryna Savickaite loading sample vials into the hot block for stable-isotope mass spectrometry. BGS © UKRI
Very small samples weighing as little as 10 micrograms — for example, a singular microfossil just visible to the naked eye — are transferred into glass vial. These vials are tightly sealed and then placed into a hot block. In the meantime, the mass spectrometer is checked and calibrated. To do this, we first introduce a reference gas against which the samples are compared. The reference gas has a known isotope composition and can therefore be used to ‘tune’ the mass spectrometer; we can calculate the isotope ratios in the sample by comparison against the reference gas.
Once we are confident the instrument is working accurately, we set it running. The run begins with a needle being inserted into the vial containing the sample. The needle has multiple holes so it can perform multiple functions without needing to be removed and re-inserted. The first hole in the needle draws out any air and creates a vacuum. It then dispenses very clean phosphoric acid (H3PO4) into the vial via a second hole. The acid dissolves the sample and liberates the carbon dioxide (CO2) it contains. As the needle does not need to be removed, the reaction can take place without atmospheric gases mixing in with the sample gas. The spectrometer is fully automated and allows us to run as many as 40 samples overnight.
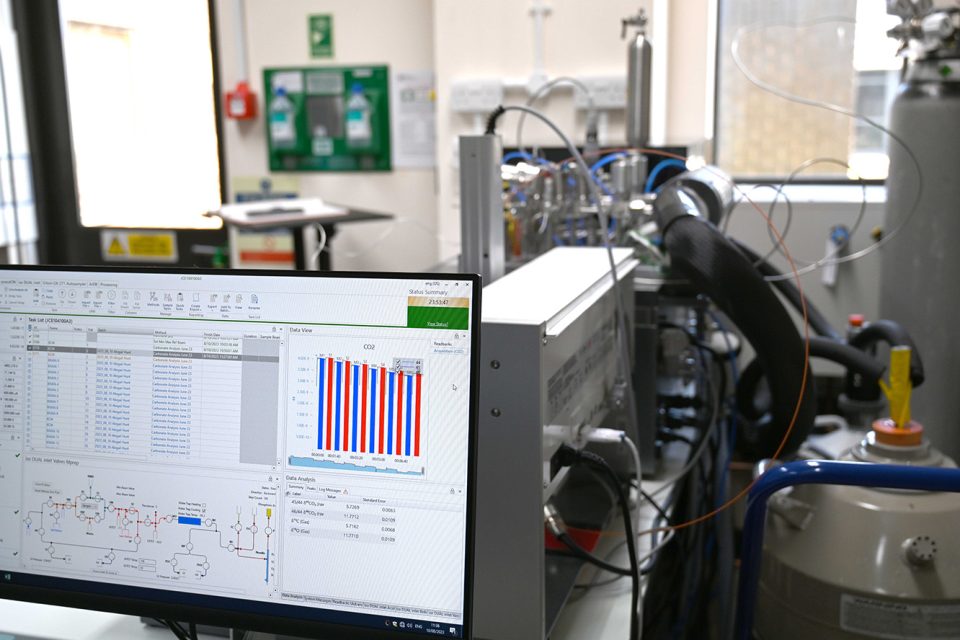
Standards of known values are placed at the beginning of the run and the raw data produced by the instrument can be viewed as soon as a sample is fully run. This allows us to check the accuracy and precision of the run and stop the instrument if any problems arise before the samples are affected. BGS © UKRI.
Once the reaction is complete, the liberated gas is taken across a ‘cold finger’, which traps water and any other gases that have also been liberated. This is a way of making sure that no impurities are included into the measurement. The mass spectrometer instrument measures how much CO2 there is in the sample and its isotope composition, which can be converted to the isotope composition of the original calcium carbonate.
Ultimately, isotope compositions can tell us about climate change in the very recent past as well as deep geological time. This can help us make accurate predictions about the effects on our climate in the future and answer important questions about climate and environmental change.

Kotryna Savickaite
Geochemistry technician

Dr Jack Lacey
Stable isotope geoscientist
Relative topics
Latest blogs

AI and Earth observation: BGS visits the European Space Agency
02/07/2025
The newest artificial intelligence for earth science: how ESA and NASA are using AI to understand our planet.

Geology sans frontières
24/04/2025
Geology doesn’t stop at international borders, so BGS is working with neighbouring geological surveys and research institutes to solve common problems with the geology they share.

Celebrating 20 years of virtual reality innovation at BGS
08/04/2025
Twenty years after its installation, BGS Visualisation Systems lead Bruce Napier reflects on our cutting-edge virtual reality suite and looks forward to new possibilities.

Exploring Scotland’s hidden energy potential with geology and geophysics: fieldwork in the Cairngorms
31/03/2025
BUFI student Innes Campbell discusses his research on Scotland’s radiothermal granites and how a fieldtrip with BGS helped further explore the subject.

Could underground disposal of carbon dioxide help to reduce India’s emissions?
28/01/2025
BGS geologists have partnered with research institutes in India to explore the potential for carbon capture and storage, with an emphasis on storage.

Carbon and oxygen isotope analysis of carbonates and the development of new reference materials
18/12/2024
Dr Charlotte Hipkiss and Kotryna Savickaite explore the importance of standard analysis when testing carbon and oxygen samples.

Studying oxygen isotopes in sediments from Rutland Water Nature Reserve
20/11/2024
Chris Bengt visited Rutland Water as part of a project to determine human impact and environmental change in lake sediments.

Celebrating 25 years of technical excellence at the BGS Inorganic Geochemistry Facility
08/11/2024
The ISO/IEC 17025 accreditation is evidence of technical excellence and reliability, and a mark of quality assurance.

Electromagnetic geophysics in Japan: a conference experience
23/10/2024
Juliane Huebert took in the fascinating sights of Beppu, Japan, while at a geophysics conference that uses electromagnetic fields to look deep into the Earth and beyond.

Exploring the role of stable isotope geochemistry in nuclear forensics
09/10/2024
Paulina Baranowska introduces her PhD research investigating the use of oxygen isotopes as a nuclear forensic signature.

BGS collaborates with Icelandic colleagues to assess windfarm suitability
03/10/2024
Iceland’s offshore geology, geomorphology and climate present all the elements required for renewable energy resources.

Mining sand sustainably in The Gambia
17/09/2024
BGS geologists Tom Bide and Clive Mitchell travelled to The Gambia as part of our ongoing work aiming to reduce the impact of sand mining.


