BGS operates and maintains a wide range of state-of-the-art laboratories and other facilities. These facilities, managed under the Laboratory Facilities Programme, underpin virtually all of our diverse range of core and commissioned research programmes.
In addition to information on capability, you will find examples of how our science facilities contribute to our research efforts.
Our science facilities

Geophysical observatories
BGS operates a series of magnetic observatories around the globe.
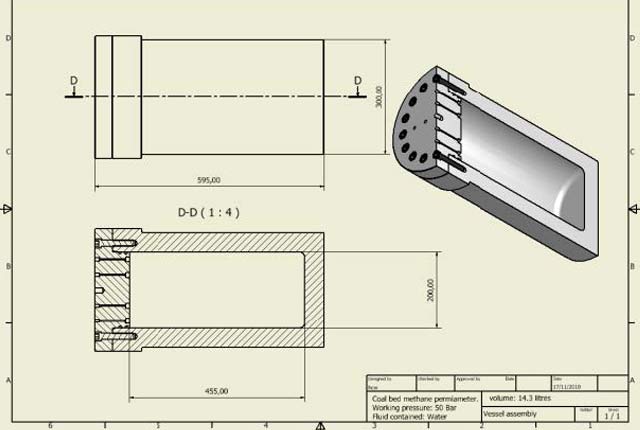
Engineering and Geotechnical Capability
Leading the development and application of field and laboratory infrastructure and long-term management of geophysical and geotechnical property data.

Centre for Environmental Geochemistry
Focusing on the use of geochemistry in research, training and teaching.
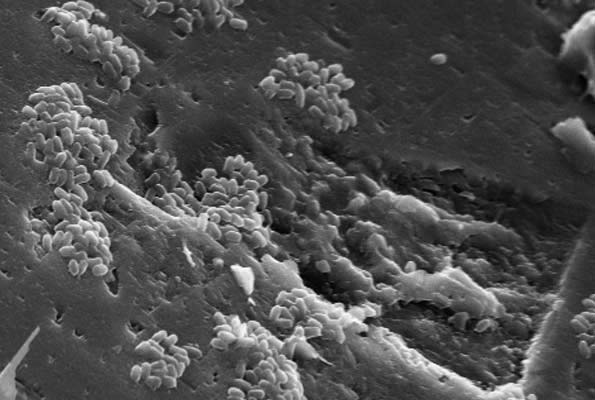
Fluid and Rock Processes Laboratory Cluster
Identifying, measuring and quantifying complex geological and environmental processes essential in the efficient utilisation of natural resources and underground spaces.

Rock Volume Characterisation Laboratory Cluster
Studying the structural and compositional characterisation of rocks and their constituent parts at all scales.
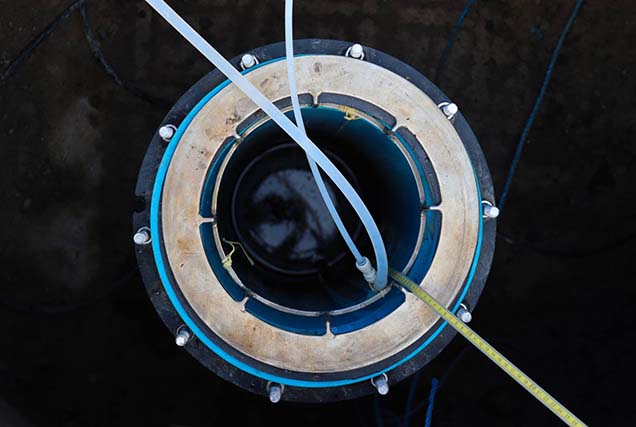
UK Geoenergy Observatories
Facilitating a step change in our understanding of geology and our relationship with the underground environment.
Related news

Dr Angela Lamb appointed as honorary professor by the University of Nottingham
02/10/2025
Dr Lamb will take up the position of honorary professor of environmental geochemistry, with a focus on collaborative research.

Gemini: a new stable isotope tool
21/08/2025
BGS’s Stable Isotope Facility has new mass spectrometer equipment for analysing carbon and oxygen isotopes from carbonates and water.

Scientists uncover secrets of Stonehenge’s mysterious cattle
20/08/2025
Cutting-edge analysis of a Neolithic cow tooth dating back to the construction of the famous landmark provides evidence of Welsh origins.

Carbon and oxygen isotope analysis of carbonates and the development of new reference materials
18/12/2024
Dr Charlotte Hipkiss and Kotryna Savickaite explore the importance of standard analysis when testing carbon and oxygen samples.

Celebrating 25 years of technical excellence at the BGS Inorganic Geochemistry Facility
08/11/2024
The ISO/IEC 17025 accreditation is evidence of technical excellence and reliability, and a mark of quality assurance.

Laboratory life: my work experience week at BGS
20/08/2024
Aspiring astrophysicist Riveen Pehesara Kumanayaka shares his experience following an A-level work placement with BGS.

Extracting formation temperatures from stalagmites
14/08/2024
BGS’s Andrew Smith explores the karstic depressions of northern Spain in the quest to create a palaeothermometer.

Harnessing global collaboration: UK/Kenya partnership in soil erosion research
31/05/2024
Collaboration between scientists is vital in today’s interconnected world to further scientific progress. In environmental research, issues such as soil erosion demand collaboration on an international scale.

Hungry like a wolf: new insights from old bones housed in the BGS museum collections
18/01/2024
BGS scientists are studying the diets of ancient British wolves and how they adapted to changing environments.

Understanding nutrients in tropical rainforests
11/01/2024
Christopher Bengt talks about carrying out research for his PhD amongst the rainforests and volcanoes of the Philippines.

Linking geochemistry and health in artisanal and small-scale gold mining in the Kakamega-Vihiga gold belt, Kenya
09/01/2024
PhD candidate Maureene Auma Ondayo is investigating major and trace element exposure in the environment in Kenya, aiming to reduce exposure of humans to toxic chemicals.

My role as a stable isotope research assistant
19/12/2023
Charlotte Hipkiss has recently taken up a new position in the National Environmental Isotope Facility at BGS and gives us a little insight into her new position.


