Weathering is the wearing down or breaking of rocks while they are in place. Weathering can be biological, chemical or physical.

The headwater of the River Teme (Afon Tefeidiad) in Mid Wales, showing biological weathering. BGS © UKRI.
Biological weathering
Biological weathering is caused by the movements of plants and animals. For example, a rabbit can burrow into a crack in a rock making it bigger and eventually splitting the rock, or a plant may grow in a crack in a rock and, as its roots grow, cause the crack to widen. Even you can be a source of weathering! Boots and shoes walking over the same patch of rock may eventually wear down the rock.
Chemical weathering
Chemical weathering describes the process of chemicals in rainwater making changes to the minerals in a rock.
Carbon dioxide from the air is dissolved in rainwater, making it slightly acidic. A reaction can occur when the rainwater comes into contact with minerals in the rock, causing weathering.
Physical weathering
Physical weathering occurs when physical processes affect the rock, such as changes in temperature or when the rock is exposed to the effects of wind, rain and waves.
Water can get into cracks in a rock and, if it freezes, the ice will expand and push the cracks apart. When the ice melts, more water can get into the larger crack; if it freezes again it expands and can make the crack even bigger.
Wind can cause weathering by blowing grains of sand against a rock, while rain and waves cause weathering by slowly wearing rock away over long periods of time.
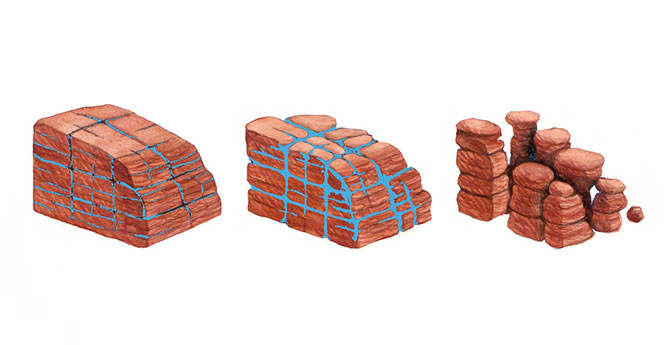
Mechanical, or physical, weathering has taken place in limestone landscapes such as the Pennines of Yorkshire. An example is ‘freeze-thaw’, where water soaks into small fissures and cracks, expands when it freezes in the winter, and physically breaks the limestone apart. BGS © UKRI.
You may also be interested in

Discovering Geology
Discovering Geology introduces a range of geoscience topics to school-age students and learners of all ages.

Geological processes
Planet Earth is dynamic with a surface that is always changing. Find out about the processes that cause these changes.

Deposition
Deposition is the laying down of sediment carried by wind, water, or ice.






