A total of 62 groundwater chemistry analyses were used to interpret the groundwater chemistry of the Carboniferous aquifers of the Midland Valley. Of these, 36 were from new groundwater samples collected for the Baseline Scotland project in September and December 2008 and one from a sample collected during an earlier Baseline Scotland sampling programme in the Borders. These were augmented with a further 25 samples collected during separate projects since 2001, which fill in many of the spatial gaps in sample collection across the study area.
The sites for the samples used in the study were chosen to be representative of groundwater in its natural state from the aquifers. However, given the history of mining in this region, a number of the sampling sites were known to be affected by mining; others may also have been affected. A small number of samples were deliberately targeted at coal mine workings.
Five aquifer groups have been identified: four chronostratigraphical groups, which, in decreasing order of age, are the Inverclyde, Strathclyde, Clackmannan and Coal Measures groups; and a fifth group incorporating waters known to be derived directly from former coal mines.

Coverage of baseline sampling in the Midland Valley against the 625K geology map of the region. BGS © UKRI.
Main findings
There are some distinct differences in groundwater chemistry between the aquifer groups. The most mineralised groundwaters (excluding mine discharges) are from the Coal Measures Group, which also have the lowest dissolved oxygen concentrations. At least some of the groundwaters identified as being from the Coal Measures Group are likely to have been affected in some way by coal mining. The least mineralised groundwaters were from the Inverclyde and Strathclyde groups.
The collection and interpretation of new groundwater chemistry data for the Carboniferous aquifers of the Midland Valley has led to the following conclusions.
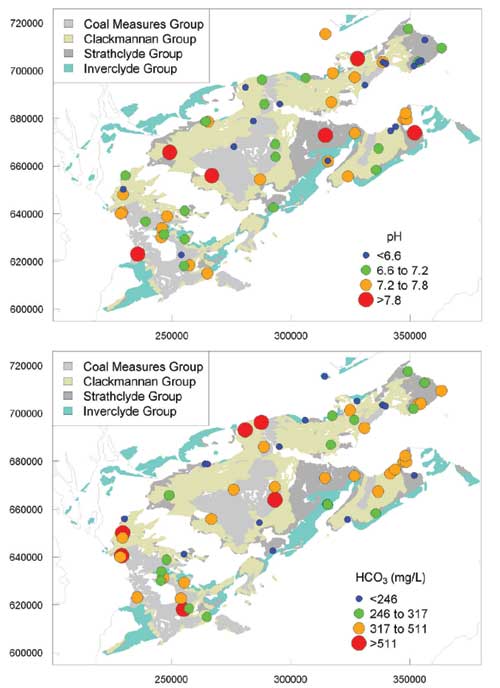
Groundwater pH
Most of the groundwater samples had a near-neutral pH (10th to 90th percentile range of 6.40 to 7.85). There was apparent little difference in pH between the different aquifer groups, indicating that the acid buffering capacity of all of the aquifers was high, most probably controlled by dissolution of carbonate minerals.
Alkalinity
Alkalinity was variable, but often high. Given the near-neutral pH of most of the groundwaters, the main species contributing to the alkalinity was likely to be HCO3. The 10th to 90th percentile range in bicarbonate concentrations was 132 to 510 mg/L The highest median bicarbonate values were for the Coal Measures Group and mine waters, although some high concentrations also occurred in the Clackmannan Group.
Groundwater types
Groundwater types encompassed a large range including Ca-HCO3, Ca-Mg-HCO3, SO4-rich types, Na-HCO3, and (rarely) Na-Cl. The groundwaters had variable calcite saturation indices but, in many cases, were close to saturation, indicating both the presence of and reaction with carbonate minerals in the aquifers.
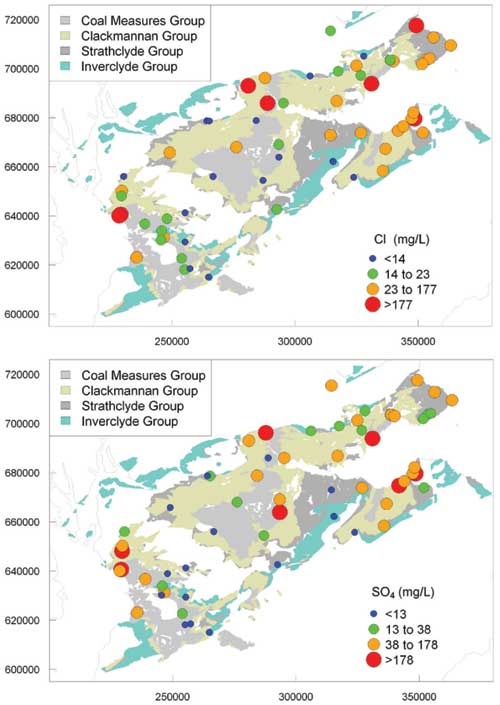
Anions
There was a large range in concentrations of the major anions. Concentrations of Cl were typically high but also highly variable, with a 10th to 90th percentile range of 7.2 to 123 mg/L. Concentrations of SO4 spanned some five orders of magnitude, with a 10th to 90th percentile range of 4.7 to 179 mg/L.
The overwhelmingly dominant processes controlling this large range are likely to be oxidation of sulfide minerals, particularly in mining-impacted waters, and sulfate reduction in the most reducing conditions. Low concentrations (<5 mg/L SO4) were usually associated with low NO3-N concentrations and often high NH4-N; all samples were recorded in the field as having a smell of H2S had SO4 concentrations of less than 5 mg/L.
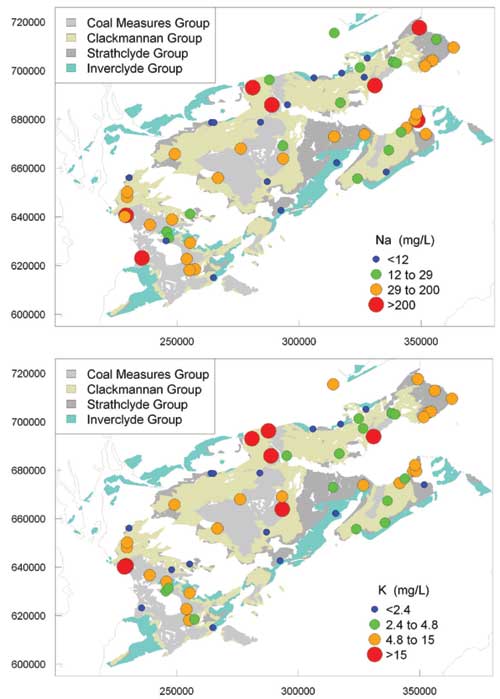
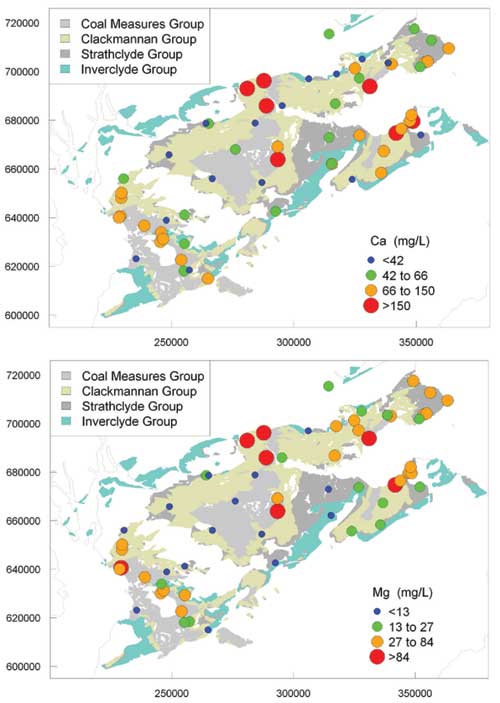
Major cations
There was large spatial variability in the concentrations of the major cations. The highest concentrations of Ca and Mg were in mine waters and some Coal Measures Group groundwater, probably due to reaction of carbonate minerals and clays, which could have been enhanced by the generation of acid during the oxidation of iron sulfide. Concentrations of Na were also often high, particularly in waters from the Coal Measures and Clackmannan groups and mine waters. Concentrations of K were correspondingly high.
The high concentrations of dissolved cations are likely to reflect the effects of extreme water/rock interactions, in some cases (particularly deep boreholes) involving long residence times in the aquifers concerned. Na was probably largely sourced from dissolution of silicate minerals and ion-exchange reactions, the latter induced by shallow, young groundwater mixing with older, saline water.
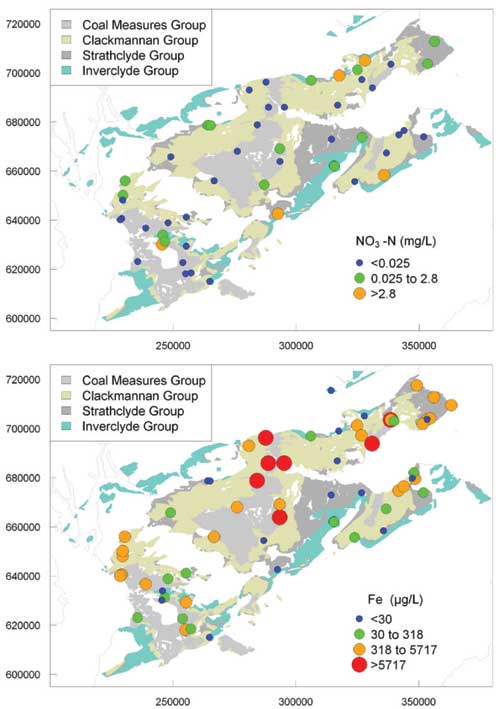
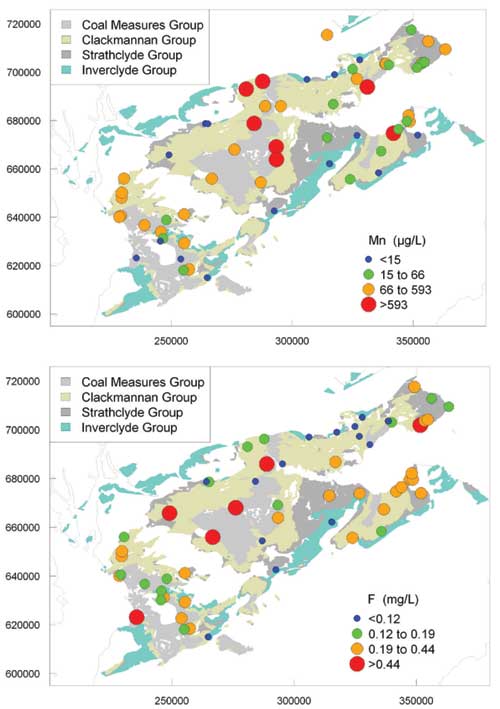
Iron and manganese
Concentrations of Fe and Mn showed large ranges, consistent with the large variations in redox status in the aquifers. The highest Fe and Mn concentrations were in groundwater from the Coal Measures Group and Clackmannan Group. Both elements were found at highest concentrations in groundwaters with low dissolved oxygen concentrations, in agreement with the findings of Homoncik et al. (2010).
Dissolved oxygen
Many of the collected groundwaters were reducing (anoxic), with 37 per cent of determined dissolved oxygen values less than or equal to 1 mg/L and 60 per cent of determined Eh values less than 250 mV. Groundwaters from the Clackmannan and Coal Measures groups were the most reducing (excluding the mine discharges).
Field sampling showed evidence that some of the groundwater sources from the Clackmannan and Coal Measures groups contained dissolved sulfide, indicating the presence of strongly reducing conditions in some parts of these aquifers. Detectable dissolved methane (CH4) was measured in a number of the samples. Samples from the Strathclyde and Inverclyde groups showed the highest dissolved oxygen concentrations. High concentrations of dissolved organic carbon (DOC) (up to 70.8 mg/L) in a few samples were also indicative of the reducing, often strongly reducing, nature of some of the groundwaters.
Nitrate and nitrite
Nitrate concentrations were highly variable with little relationship to land use, showing the strong control that redox conditions have on groundwater chemistry. It also indicated that denitrification, which occurs under reducing conditions, had been important in many of the groundwaters.
Nitrite was detectable in a few low-NO3 groundwaters and, as an intermediate reaction product, is an additional indication of denitrification.
Phosphorus
The highest concentrations of phosphorus were in the Coal Measures and Clackmannan groups, with sources likely to include dissolution of phosphate minerals, desorption from iron oxides under reducing conditions and degradation of organic matter. Agricultural pollution may have been contributory in some cases, but some of the outlier phosphorus concentrations occurred in the more reducing (and mining-affected) groundwaters, which would be consistent with a natural (mineral, organic matter) origin.
Dissolved gases
Dissolved carbon dioxide (CO2) was measured in selected groundwaters and the results indicate that pCO2 is often high, controlling carbonate equilibrium. Dissolved CH4 was present at 10 μg/L or less in approximately half the samples measured. Such values are typical of those found in major aquifers such as Cretaceous Chalk and the Permo-Triassic Sherwood Sandstone Group.
The remainder of the samples ranged up to about 10 mg/L. Waters with dissolved CH4 concentrations higher than 1.5 mg/L can in principle give rise to explosive atmospheres in confined situations, such as buildings and excavations.
Mining
Mining activity has had a major impact on groundwater quality in the region. This is particularly the case for the samples collected directly from discharges (pumped or gravity flows) from abandoned coal mines. Six samples of groundwater were sourced directly from abandoned coal mines and showed a wide range of chemical types.
The mine-discharge waters are typical strongly mineralised, with high electrical conductivity (SEC) values and particularly high concentrations of HCO3, Ca, SO4, Fe and Mn. The waters were generally low in dissolved oxygen and showed the evidence of pyrite oxidation within the mines. The pH values were generally well buffered and alkalinity was high, indicating significant reaction with carbonate material in the aquifers.
Five of the Baseline Scotland samples (two from the Clackmannan Group and three from the Coal Measures Group) were collected from sources known or strongly suspected to intercept abandoned mine workings. Although none of these sources was specifically abstracting mine waters, it is likely that at least some of the groundwater pumped from them derived from the mine workings. These five samples tended to be more mineralised than samples thought unlikely to be affected by mining, although generally not as mineralised as the ‘mine’ waters. This was particularly the case for concentrations of HCO3, K, SO4, Fe and Mn, and for the SEC of the groundwater.
Average Ca concentrations in these five samples were as high as those in the ‘mine’ waters. Average concentrations of Cl, Mg and Na in these samples were similar to those in samples thought unlikely to be impacted by mining.
Interpretations in terms of groundwater flow
Hydrochemical data and information on groundwater residence times can help give insight into groundwater flow in aquifers across the Midland Valley. Many of the groundwater samples from the studied aquifers were from deep boreholes, where groundwater was often present under confined conditions and within multilayered aquifers.
Groundwater flow was dominated by flow within fractures. Analysis of dissolved gases and stable isotopes indicated that the groundwaters contained a high proportion of relatively old water, recharged more than 35 years ago, and a significant proportion of water was recharged more than 60 years ago. However, there is little evidence of the existence of palaeowater (older than 10 000 years).
Many of the groundwaters showed evidence of having been affected by ion-exchange reactions (Na for Ca exchange), which suggests that young, shallow groundwater has mixed with older, more mineralised water.
Summary statistics
Coal Measures Group
Groundwaters from the Coal Measures Group were generally of bicarbonate type, either dominated by Na or with no dominant cation. Average alkalinity values were the highest of all the hydrogeological units sampled, including the ‘mine’ waters. There was a large range in SEC values and the median value was the highest for all the groups except ‘mine’ waters.
The waters were generally anoxic and slightly acidic to near-neutral. They typically had moderate concentrations of the major cations Ca, Mg, Cl and SO4 and high concentrations of Na relative to the other aquifer groups. In most cases, the calcite saturation index showed the groundwaters were close to saturation.
There was a large range in Fe and Mn concentrations but they were usually high, with the highest average of any group except the ‘mine’ waters.
Clackmannan Group
Groundwaters from the Clackmannan Group were generally either of Ca-Mg-HCO3 type, Na type or had no dominant anion; a few showed a cationic dominance of Na. The groundwaters typically had high HCO3 concentrations, a near-neutral pH and generally low dissolved oxygen. SEC values showed a wide range but a moderate average.
The waters generally had moderate to high concentrations of the major cations Ca, Mg, Ba and Cl. Concentrations of SO4 were the highest of all the hydrogeological units except ‘mine’ waters. Most of the samples were significantly undersaturated with respect to calcite.
Concentrations of Fe and Mn were moderate to high, with variability due to the redox conditions in the aquifer.
Inverclyde Group
Most of the groundwaters from the Inverclyde Group were of Ca-HCO3 type. They typically had moderate HCO3 with a near-neutral pH. Dissolved oxygen concentrations were generally low but most of the waters were not anoxic. SEC values were typically moderate.
The groundwaters typically had relatively low concentrations of the cations Na, Cl and SO4 and moderate concentrations of Ca and Mg.
In most cases, the calcite saturation index showed the groundwaters were close to saturation. Concentrations of Fe and Mn were typically relatively low, reflecting the generally oxic nature of the groundwaters from this group.
Strathclyde Group
Most of the groundwaters sampled from the Strathclyde Group were of Ca-Mg-HCO3. Some samples were more dominated by Na with no anionic dominance. The groundwaters typically had moderate HCO3 concentrations with near-neutral pH. Dissolved oxygen concentrations were usually low but most of the waters were not anoxic. SEC values were generally moderate to high.
The waters typically had moderate concentrations of the cations Ca, SO4, Na, Cl and Mg. In about half of the samples the calcite saturation index showed the groundwaters were close to saturation or saturated with respect to calcite; the other half were undersaturated.
Fe concentrations were relatively low on average but showed a wide range. Mn concentrations were typically moderate.
Maps of regional variation in selected ion concentrations
Full report
You can download the Baseline Scotland: groundwater chemistry of the Carboniferous sedimentary aquifers of the Midland Valley report.
Further reading
Homoncik, S, MacDonald, A M, Heal, K V, Ó Dochartaigh, B É, and Ngwenya, B T. 2010. Manganese concentrations in Scottish groundwater. Science of the Total Environment, Vol. 408, 2467–2473. DOI: https://doi.org/10.1016/j.scitotenv.2010.02.017