Excessive manganese (Mn) concentrations can result in:
- metallic-tasting water
- staining of clothes, dishes and products such as paper or plastics
- reduced water pressure and flow in pipes from accumulation of Mn oxides
(Sly et al., 1990)
Mn oxides also have an adverse effect on human health. In adults, exposure to elevated Mn concentrations in drinking water has been associated with manganism, a Parkinson’s disease-like disorder, and, in children, intellectual function issues and hyperactive behaviour. Because of the association between Mn intake and neurological effects, the World Health Organization (WHO) current guideline concentration for Mn in drinking water is 0.4 mg l-1 (World Health Organisation, 2008).
Controls on manganese in groundwater
The occurrence and concentration of Mn in groundwater are controlled by many factors, the main ones being:
- rock geochemistry
- water chemistry
- microbiological activity
Some rock types, such as mafic and ultramafic igneous rocks, shale, greywacke and limestone, contain high concentrations of Mn, which can lead to elevated concentrations in soil and sediment through weathering processes. Water chemistry, in particular pH, redox potential (Eh), dissolved oxygen (DO), and dissolved organic carbon (DOC), is instrumental in mobilising Mn and controlling its speciation and concentration in the water environment.
Research on manganese in Scotland
Research using data by Baseline Scotland (Hominik et al. 2010) assessed the concentrations of Mn in Scottish groundwater between different aquifer types, and examined the controls on elevated Mn concentrations in groundwater.
This research developed a quality-controlled dataset of groundwater Mn concentrations across Scotland, with data for 475 sites.
Results
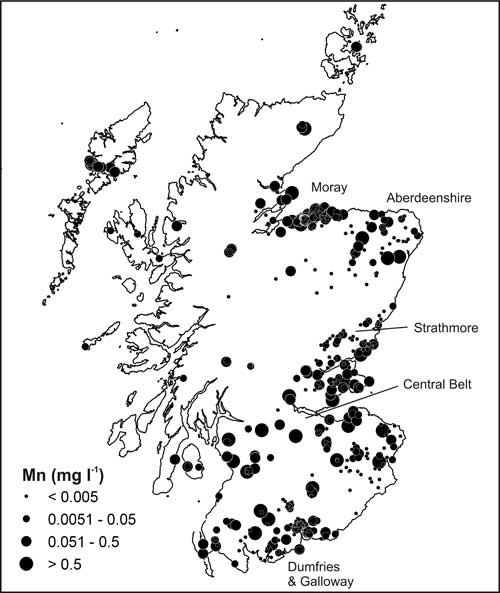
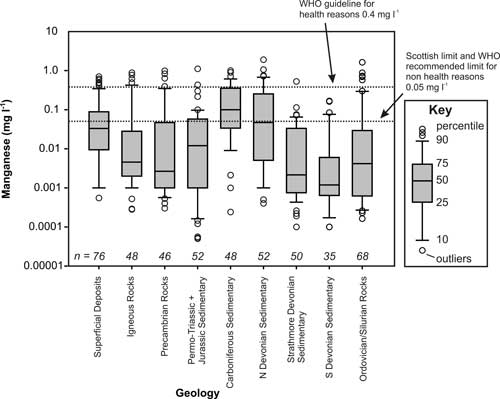
Analysis of the dataset showed that elevated Mn concentrations (more than 0.05 mg/L) occurred in 30 per cent of the groundwater samples (Figure 1), particularly in superficial, Carboniferous and northern Devonian aquifers (figures 2 and 3), and 9 per cent of sites had concentrations above the WHO health drinking water limit (0.4 mg/L).
The principal controls on Mn concentrations in groundwater in Scotland are redox conditions and pH, with some influence from Fe behaviour. Redox conditions exercise the strongest control.
The results highlight the potential risk of exposure to excessive Mn in drinking water for consumers using private water supplies and indicate the need for appropriate methods for sampling and testing groundwater for Mn, not only in Scotland but worldwide.
Further reading
Homoncik, S, MacDonald, A M, Heal, K V, Ó Dochartaigh, B É, and Ngwenya, B T. 2010. Manganese concentrations in Scottish groundwater. Science of the Total Environment, Vol. 408, 2467–2473. DOI: https://doi.org/10.1016/j.scitotenv.2010.02.017
MacDonald, A M and Ó Dochartaigh, B É. 2005. Baseline Scotland: an overview of available groundwater chemistry data for Scotland. British Geological Survey Commissioned Report, CR/05/239N. (Nottingham, UK: British Geological Survey.) Available: https://nora.nerc.ac.uk/id/eprint/11335/
MacDonald, A M, Robins, N S, Ball, D F and Ó Dochartaigh, B É. 2005. An overview of groundwater in Scotland. Scottish Journal of Geology, Vol. 41, 3–11. DOI: https://doi.org/10.1144/sjg41010003
Robins, N S. 2002. Groundwater quality in Scotland: major ion chemistry of the key groundwater bodies. Science of the Total Environment, Vol. 294(1–3), 41–56. DOI: https://doi.org/10.1016/S0048-9697%2802%2900051-7
Sly, L J, Hodgkinson, M C, and Arunpairojana, V. 1990. Deposition of manganese in a drinking water distribution system. Applied Environmental Microbiology, Vol. 56, 628–639. DOI: https://doi.org/10.1128/aem.56.3.628-639.1990